【Cell】转座子“跳跃”过程调控机制
Cell:转座子“跳跃”过程调控机制
2013/11/25 22:58:11

“跳跃基因”的正式名称应该为转座子或转座元件,是一段能够插入到基因组新位置的DNA片段。人类基因组包含了大量古老的跳跃基因的垃圾片段,由于细胞要抑制跳跃基因的胡作非为,细胞进化出了针对转座子的调控机制。近期发表在Cell杂志上的文章揭示了调控跳跃基因的新机制。
大多数跳跃基因都会产生突变并不再”跳跃”,但是有一个基因例外–L1基因。该基因成功的复制自己,占据了人类DNA的20%。尽管很多拷贝发生突变,还是有很多拷贝处于活跃状态,这引起了遗传学家的兴趣。
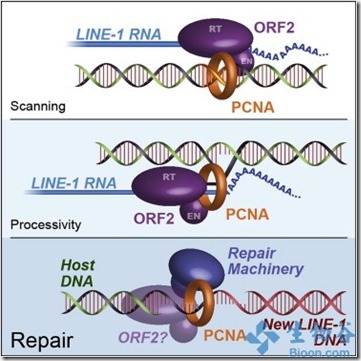
Lixin Dai博士称人类细胞进化出限制跳跃基因活性的机制,因为它们越活跃就越容易破坏重要的基因,造成严重影响。Dai博士领导研究团队研究了L1的调控机制,发现两种类型的L1蛋白与RNA形成核糖核蛋白复合物,L1就是依靠该复合物进行”跳跃”。
为了进一步研究跳跃基因的调控机制,科学家分析了与该核糖核蛋白相互作用的蛋白,发现有37个蛋白与核糖核蛋白相互作用,于是科学家选择了两个蛋白进行进一步的研究。一个是UPF1,与质量控制有关,该蛋白监控RNA,会销毁发生错误的RNA。Dai博士称,当抑制UPF1功能的时候细胞会产生更多的L1的RNA和蛋白质。
另一个蛋白是PCNA,该蛋白帮助DNA复制。科学家发现PCNA能够与核糖核蛋白复合物中L1蛋白的一段重要序列结合,当科学家改变该结合序列后,L1不再”跳跃”。 Dai博士称,UPF1的作用是抑制L1活性,而PCNA看起来更像是协助L1进行跳跃。
Dai博士称我们的研究揭示了跳跃基因和细胞之间的博弈,表明细胞如何抑制跳跃基因的活性。我们将继续研究跳跃基因和细胞之间的”军备竞赛”。
Affinity Proteomics Reveals Human Host Factors Implicated in Discrete Stages of LINE-1 Retrotransposition
doi: 10.1016/j.cell.2013.10.021
Martin S. Taylor, John LaCava, Paolo Mita, Kelly R. Molloy, Cheng Ran Lisa Huang, Donghui Li, Emily M. Adney, Hua Jiang, Kathleen H. Burns, Brian T. Chait, Michael P. Rout, Jef D. Boeke, Lixin Dai.
LINE-1s are active human DNA parasites that are agents of genome dynamics in evolution and disease. These streamlined elements require host factors to complete their life cycles, whereas hosts have developed mechanisms to combat retrotransposition’s mutagenic effects. As such, endogenous L1 expression levels are extremely low, creating a roadblock for detailed interactomic analyses. Here, we describe a system to express and purify highly active L1 RNP complexes from human suspension cell culture and characterize the copurified proteome, identifying 37 high-confidence candidate interactors. These data sets include known interactors PABPC1 and MOV10 and, with in-cell imaging studies, suggest existence of at least three types of compositionally and functionally distinct L1 RNPs. Among the findings, UPF1, a key nonsense-mediated decay factor, and PCNA, the polymerase-delta-associated sliding DNA clamp, were identified and validated. PCNA interacts with ORF2p via a PIP box motif; mechanistic studies suggest that this occurs during or immediately after target-primed reverse transcription.