植物碳积累途径完全阐明 SWEET! The Pathway Is Complete
植物碳积累途径完全阐明[SWEET! The Pathway Is Complete]
2012/06/30 21:04:49
Science 13 January 2012: 335(6065): 173-174
SWEET! The Pathway Is Complete
David M. Braun
Photosynthesis in plants leads to the accumulation of carbohydrates (e.g., sugars, starch), upon which all terrestrial life depends. In most plants, sucrose is the principal carbohydrate transported long-distance in the veins to support the growth and development of roots, flowers, fruits, and seeds. Sucrose can be directly stored in specialized tissues, such as fruits or the stems of sugarcane and sweet sorghum, or it can be converted into starch in cereal seeds and potato tubers. Thus, proper control of carbohydrate partitioning is fundamental to crop yield and human nutrition and to the development of plant-based biofuels. Given the importance of this process, it may come as a surprise that until now, we did not understand the entire pathway for the export of sucrose from leaves. On page 207 of this issue, Chen et al. (1) identify and characterize the long-sought missing player in sucrose transport, the sucrose effluxer.
植物光合作用导致碳水化合物(如糖,淀粉)的积累,所有陆栖生命皆依赖于此。大多数植物中,蔗糖是长距离运输的最主要碳氺化合物,供应根、花、果实、以及种子的生长与发育。蔗糖可以直接贮存于某些组织,像果实或者甘蔗与甜高粱的茎,也可转化为谷类种子与土豆块茎中的淀粉。因此,适当控制碳水化合物的分配对于作物产量与人体营养,乃至发展基于植物的生物燃料,是很必要的。考虑到此过程的重要性,令人惊奇的是,时至现在,我们依然没有完全理解蔗糖从叶片输出的途径。在本期(科学-335卷-6065期)的第207页,Chen等鉴定并阐明了长久以来所探究的蔗糖运输中缺失角色——蔗糖输出器。
Carbon assimilation in mature leaves results in a surplus of carbohydrates, which are exported through the veins to nonphotosynthetic tissues (2–7). Sucrose is synthesized in leaf mesophyll cells and diffuses cell-to-cell through plasmodesmata (conduits spanning the cell wall and connecting adjacent cells) toward the vein (see the figure). Within the veins, the phloem is the specialized tissue involved in long-distance sucrose transport. The phloem contains three cell types: parenchyma cells, companion cells, and sieve elements. In the majority of crop plants, the companion cells and sieve elements are not connected by plasmodesmata to the other cells in the leaf; therefore, sucrose must be effluxed from the phloem parenchyma cell to the cell wall space (apoplast) before being imported into the companion cells and/or sieve elements by sucrose transporters located on their plasma membranes (2–7). The portions of the sucrose transport pathway from the mesophyll cell to the phloem parenchyma cell, and from the apoplast into the companion cell and sieve element, have been well characterized. However, the mechanism of sucrose efflux into the apoplast, the last unresolved step in the sucrose phloem loading pathway, remained a mystery (8). The sucrose effluxer was finally identified by Chen et al. through an elegant approach that combined cell biology, biochemistry, genomics, and genetics.
成熟叶片中碳的积累引起碳水化合物的盈余,这部分碳氺化合物通过叶脉输出到不能进行光合成的组织。蔗糖合成于叶肉细胞,通过细胞间的胞间连丝(胞间连丝是跨越细胞壁并连接相邻细胞的通道)在扩散至叶脉(见下图)。在叶脉中,韧皮部是负责长距离蔗糖运输的分化组织。韧皮部有三种细胞类型:薄壁细胞、伴细胞和筛管分子。对于大多数作物,伴细胞与筛管分子并没有通过胞间连丝与叶片其它细胞相连;因此,蔗糖必须在进入伴细和/或筛管分子之前通过质膜上的蔗糖转运子,由韧皮部薄壁细胞输出至细胞壁空间。从叶肉细胞到韧皮部薄壁细胞,以及从质外体进入伴细胞和筛管分子,这两部分蔗糖转运途径已被阐明。然而,蔗糖输出至质外体的机制——韧皮部装载蔗糖途径最后一个未解决的步骤,依然是个谜。蔗糖输出子终于被Chen等通过一种简洁的方法,结合细胞生物学、生物化学、基因组学和遗传学,最终被鉴定出。
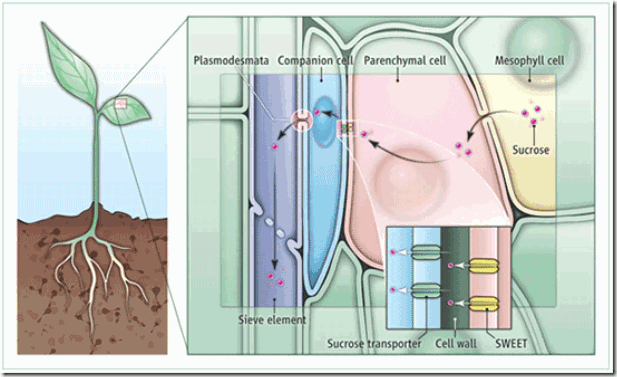
Sucrose partitioning in plants.
Sucrose is synthesized in leaf mesophyll cells and diffuses through plasmodesmata into phloem parenchyma cells. SWEET proteins facilitate sucrose efflux into the cell wall (apoplast). Sucrose transporters import sucrose into companion cells and/or sieve elements. Sucrose is transported through sieve elements out of leaves to nonphotosynthetic tissues, such as roots, stem, and fruits.
蔗糖合成于叶片叶肉细胞,通过胞间连丝扩散至韧皮部薄壁细胞。SWEET蛋白促进了蔗糖进入细胞壁空间(质外体)。再由蔗糖转运子将蔗糖转运至伴细胞和\或筛管分子。蔗糖经由筛管分子转运出叶片,进入非光合成组织。例如:根部、茎和果实。
CREDIT: Y. HAMMOND/_SCIENCE_
A key that enabled this breakthrough was the development of fluorescence resonance energy transfer (FRET) optical sensors that could be used in cells to report the sugar concentration in the cytoplasm (9_, _10_). A sugar-binding protein domain was placed between variants of cyan fluorescent protein and yellow fluorescent protein. When the sensor protein binds sugar, it undergoes a conformational shift that alters the fluorescence, such that a change in the amount of fluorescence emitted can be used to monitor changes in sugar concentration. By expressing such an optical sensor for glucose or sucrose, root cells were observed to rapidly transport the sugars across cellular membranes in response to concentration gradients (11_). This led to the hypothesis that novel membrane proteins mediate sugar transport because the expression patterns and biochemical transport properties observed were inconsistent with known sugar transporters.
此项研究突破的关键之处在于应用了荧光共振能量转移(FRET)光学传感器,此技术用于监测胞质蔗糖浓度。一种糖蛋白结合蛋白质功能域被置于青绿色荧光蛋白和黄色荧光蛋白变体之间。传感器蛋白在结合糖类时发生构象变化,此构象变化改变荧光,散发荧光强度的变化可用于监测糖浓度的变化。通过表达这种葡萄糖或者蔗糖光学传感器,可观察到根部细胞经由质膜快速顺浓度梯度转运糖类。从而得出结论:新膜蛋白介导了糖转运,因为表达类型以及所观测到的生化转运特性与已知的糖类转运子不一致。
To identify these unknown proteins, Chen et al. previously used a human cell line to coexpress the glucose sensor and a collection of Arabidopsis proteins containing multiple membrane-spanning domains (12_). The authors found that a specific SWEET protein could take up glucose from the cell culture medium. SWEETs are membrane proteins that transport glucose molecules across a membrane down a concentration gradient. Phylogenetic analysis revealed that _SWEET genes are evolutionarily conserved from plants to humans. There are 17 SWEET genes in Arabidopsis and 21 in rice. Intriguingly, different bacterial or fungal pathogens obtain carbohydrates from plants by increasing the expression of different plant SWEET genes (_12_).
为了鉴定这些未知蛋白,Chen等先前使用了人类细胞系,用于共表达葡萄糖传感器与一系列含有多种跨膜功能域的拟南芥蛋白。最终发现一种特异的SWEET蛋白可以从细胞培养基中摄取葡萄糖。SWEET是一种顺浓度梯度转运葡萄糖跨膜的膜蛋白。进化分析表明,SWEET基因从植物到人类进化保守。拟南芥有17个SWEET基因,而水稻有21个。令人好奇的是,某些细菌或者真菌病原体也可通过表达不同的植物SWEET基因来获取碳水化合物。
Chen et al. determined that AtSWEET11 and 12 (and OsSWEET11 and 14 in rice) transport sucrose in Arabidopsis (1_). Both transporters localize to the plasma membrane and are expressed in a subset of leaf phloem parenchyma cells, proximal to the companion cells and sieve elements. Mutations in either the _AtSWEET11 or 12 genes produced no obvious phenotypes, but double mutants (atsweet11;12) showed moderate defects in sucrose phloem transport and an excessive accumulation of carbohydrates in the leaves. A third gene, AtSWEET13, showed increased expression in the atsweet11;12 double mutant background and may partially compensate for their function. Hence, these SWEET genes are genetically redundant, which likely explains why earlier genetic screens failed to identify the efflux step. Collectively, the data demonstrate that the AtSWEET11 and 12 genes encode the missing link in sucrose phloem loading, the sucrose effluxer.
Chen等测定拟南芥中AtSWEET11与12转运蔗糖(水稻中为OsSWEET11与14)。两种转运子定位于质膜,在一部分靠近伴细胞和筛管分子的叶片韧皮部薄壁细胞中表达。缺失_AtSWEET11或_12_基因的拟南芥突变体没有明显的表型变化。但是双突变体(_atsweet11;12)_表现出韧皮部蔗糖转运的适度缺陷,以及叶片中碳水化合物的积累。另一个基因,AtSWEET13,在_atsweet11;12 双突变体中表达增加,部分补偿了缺失基因功能。因此,这些_SWEET_基因是遗传性冗余,这很大程度上揭示了先前鉴定输出步骤中遗传筛选的失败。这些数据共同证实了_AtSWEET11_和_12_基因编码韧皮部蔗糖装载中缺失的一环——蔗糖输出子。
The identification of SWEET proteins as sucrose facilitators raises a number of questions. Are the regulation and localization of SWEETs and sucrose transporters coordinated to maximize phloem loading efficiency and minimize any potential loss of sucrose to the apoplast (and thereby to pathogens)? Additionally, AtSWEET11 and 12 are expressed in most Arabidopsis tissues; what other roles beyond phloem loading might they play? One possibility is that they may function in sucrose efflux to seeds (13). Another is that during long-distance transport, SWEETs may facilitate the “leakage” of sucrose from the phloem to nourish adjacent stem tissues (14_). If so, manipulating _SWEET expression could enhance carbohydrate delivery to developing seeds to increase yield, or it could increase the sucrose concentration in the storage cells of sugarcane or sweet sorghum stems to improve biofuel production.
SWEET蛋白作为蔗糖输出子的鉴定引起了若干问题。SWEET与蔗糖转运子的调节与定位对于最大化韧皮部装在效率以及最小化向质外体(或者病原体)运输的潜在蔗糖损失相协调?另外,AtSWEET11_和_12 表达于拟南芥多数组织中;除了韧皮部装载其它的作用是什么?一种可能是它们可能在蔗糖输出至种子过程起作用。另一种可能是,在长距离运输中,SWEET可能促进蔗糖由韧皮部的“渗漏”,以便滋养相邻的茎组织。如果是这样,控制SWEET表达可以增加碳水化合物向正在发育的种子的传递,以增加产量,或者,也可以增加甘蔗或者甜高粱贮存细胞中蔗糖浓度,以改善生物燃料生产。
Source from somewhere